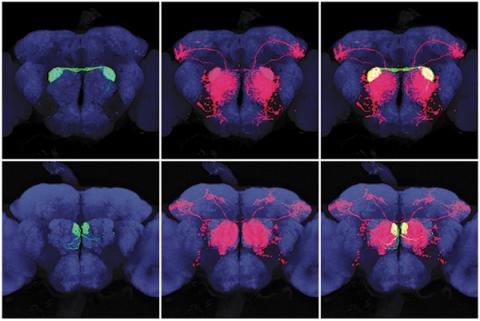
Gilad Barnea remembers his “Eureka” moment. After 20 years of work on his “trans-Tango” project, he was sitting in his Sidney Frank Hall office at Brown when a student in his lab rushed in with a picture that showed it was finally working.
Considerably more work remained before the discovery would be formally announced with publication in the journal Neuron in the fall of 2017, but the goal was in sight.
After years of efforts aimed at finding a powerful new way of studying neural circuits, “that was an amazing moment,” recalled Barnea, an associate professor of neuroscience. The new technology reveals which neurons are connected with others and makes it possible to trace brain circuits, which is key information in understanding and treating disease. It is recognized as a tool that could lead to many more discoveries, both in techniques and therapies. “Our system endows you with genetic accessibility to neural circuits based on connectivity,” Barnea said.
Now that the basic technique is proven, work with trans-Tango is expanding. After years of research using fruit flies, it is now being tried with mice, zebrafish, and chicks. And new versions of the technique are being tested to study cancer and also the immune system. In each new use, it will need to be “tweaked” to operate correctly, yet Barnea is confident that trans-Tango “is bound to give us insights into disorders of the nervous system.”
Many researchers might have given up years earlier, Barnea said, but he kept going with his “obsession,” first as a postdoc at Columbia University and then coming to Brown halfway into his two decades of work.

“Brown was perfect for this,” Barnea said, as the University provided the needed intellectual environment and crucial internal funding at key points prior to getting a succession of external grants. He said Brown has been the source of “spectacular undergraduates” for his lab (five of whom were listed as authors on the key published paper), along with graduate students, postdocs, and supportive faculty colleagues.
Persistence like Barnea’s is not unusual in brain science. The study of the brain and its relationship to cognition, behavior, and disease is often described as the “last frontier” in biomedical science, and many of its vital questions have remained extremely difficult to answer.
In 2018, Brown received one of the largest gifts in its history to support brain science research. Aiming to quicken the pace of scientific discovery about the brain and find cures to some of the world’s most persistent and devastating diseases, such as ALS and Alzheimer’s, Robert J. Carney ’61 and Nancy D. Carney gave Brown $100 million for the brain science institute, and it was renamed in their honor.
“This is a signal moment when scientists around the world are poised to solve some of the most important puzzles of the human brain,” said Brown President Christina Paxson. “This extraordinarily generous gift will give Brown the resources to be at the forefront of this drive for new knowledge and therapies. We know that discoveries in brain science in the years to come will dramatically reshape human capabilities, and Brown will be a leader in this critical endeavor.”
Diane Lipscombe, director of the Carney Institute and a professor of neuroscience, called the Carneys’ gift “lifeblood to driving innovation and discovery.” It will be used to accelerate hiring of leading faculty, supply seed funding for high-impact new research, and fund essential new equipment and infrastructure in technology-intensive areas of exploration.
Judy Liu is the kind of researcher that the Carney Institute plans to build its growth around.
A physician-scientist looking for an epilepsy cure, she had a plan. Liu thought examining patient samples would hold the key to understanding severe epilepsy. In fact, her team had identified a change in the CLOCK protein in brain tissue that generates seizures.
The next step was to develop an animal model to study the effect of decreased CLOCK on seizures. When she was examining the data for the mice engineered for this purpose, she had mountains of information. Trying to streamline her analysis, she focused on the mice during their waking hours and saw no evidence of seizures or a connection with epilepsy.
Then she had an aha moment, realizing she had to look at the complete sleep-wake cycle. When she examined the sleep periods of mice, she said, “It was so unbelievable.” In sleep, the link between lack of CLOCK and seizures suddenly became vivid. It was a turning point in her research, and her findings have given hope for a new way to develop treatment for some of the most severe cases of the disabling disorder.
“If we start worrying about how long it will take, we’ll never get started,” Liu said. “Neuroscience and particularly neurological disease have eluded biomedical researchers in finding cures.” While it won’t be easy or quick, Liu said she’s hopeful that progress can be made on epilepsy because of new insights about CLOCK. She said a strategy for further research could be to deliver a drug that compensates for the lack of CLOCK, directly in a specific part of a patient’s brain.
Liu conducted four years of research on the CLOCK protein while at the Children’s National Medical Center in Washington, D.C., and, shortly before it was published in Neuron in October 2017, she came to Brown as an assistant professor of neurology. Liu said she wanted to be at Brown and the Warren Alpert Medical School because of “the amazing basic science community,” and already she’s advancing her work in many new directions. The ability to collaborate at Brown has been fruitful right away, as she has seen that lab space and researchers are not in silos. “Having world experts in neuroscience around me will make things go faster,” she said.
Carney’s connections are highly active through the medical school and also throughout the University. One of Brown’s often cited strengths in brain science is the breadth of the faculty and research connections—with up to 45 labs across campus engaged in brain-related research and more than 180 affiliated professors in departments ranging from neurology and neurosurgery to engineering and computer science.
Undergraduates are also closely knitted into brain science. About a quarter of all Brown undergraduates take the Introduction to Neuroscience course, and many work as research assistants in brain-related campus labs.
Christopher Moore has for eight years been following in the footsteps of Aristotle, working to create bioluminescent tools for neuroscience, and students have been integral to his lab. Bioluminescent light is the glow that can be observed in organisms like fireflies and jellyfish, and it has long fascinated scholars.

More than 2,000 years ago, Aristotle recognized that the generation of light by creatures, and their ability to tolerate bright light, was remarkable. Moore, professor of neuroscience and Carney Institute associate director, and his team at Brown are aiming to use the same kind of chemical light generation to achieve two defining goals of neuroscience: The widespread measurement of brain activity and the control of these same neurons. The impact of bioluminescent tools could ultimately aid in diseases — in Parkinson’s, for instance, the light could make cells less hyperactive, helping treat patients.
Moore and Brown recently received a grant of up to $9.2 million from the National Science Foundation’s NeuroNex program to lead a national center to disseminate such tools, in collaboration with Central Michigan University and the Scintillon Institute. The group is spreading knowledge through workshops and other avenues of the burgeoning global “open science” movement, putting Brown at the forefront of efforts to break down information barriers.
While discoveries along the way have been important, Moore seems proudest of two instances of collaboration that have dramatically driven the project.
The first pivotal joint effort came in 2011, when Moore was new to Brown. While they had come up with the initial ideas, he and a postdoc knew their lab was not equipped to do the needed testing. They decided that Lipscombe, a senior colleague, was best positioned to assist them, but they were nervous about making such a request of an established colleague. Moore recalls walking down the hall and shyly knocking on her door. After he spoke, Lipscombe calmly said, “The way to test it would be this.” And, Moore remembers, “she had all sorts of ideas.” Within a week, the two labs were working together and had initial evidence that bioluminescence could control cells. It was “the Brown way,” he came to see, where collaboration is the norm.
Another critical partnership developed the following spring. A key early collaborator with Moore was a colleague at Duke, who heard that another Duke researcher, Ute Hochgeschwender, was working on something similar. Moore spent money from a grant to fly Hochgeschwender to Providence to give a lab talk. “She was just fantastic,” he said, and they decided to collaborate rather than compete, an enduring relationship that continues with Hochgeschwender now at Central Michigan University.
In 2018, many significant research steps were taken at Carney—including those led by Barnea, Liu, and Moore—and collaboration was an essential thread, Lipscombe said. She said collaboration infuses and elevates work throughout Carney.
In the fall of 2018, the National Institutes of Health announced a $12 million renewal for the next five years for the Center for Central Nervous System Function, part of the Carney Institute and led by neuroscience professor Jerome Sanes. The grant will launch five new research projects across many departments and further develop analysis tools to advance brain science at Brown.
“There are literally no boundaries for working across disciplines, which is where the exciting things happen,” Lipscombe said. “We don’t see boundaries.”