The interdisciplinary study, published online recently in the Journal of Neuroscience, points to a potential new site that interventions for ALS could target, according to Kristi Wharton, professor of biology and principal author of the study.
A dysfunction in sensory feedback may be responsible for the degeneration of motor neurons characterized by ALS. These effects, however, may be preventable or partially reversible: through the activation of bone morphogenetic protein (BMP) signaling, the researchers were able to restore motor function in fly models.
The study documents the behavior of fruit flies in various stages of development. The flies carry a mutation in the gene SOD1, which is found in about 20 percent of people with the genetic form of ALS, according to the Muscular Dystrophy Association.
Pharates – or flies that are fully formed but have yet to emerge from their pupal cases – must break out of their cases, a process called eclosion, by using abdominal muscle contractions. However, none of the mutants successfully eclosed. The flies showed disorganized muscle structure and had fewer synaptic boutons, or sites at the end of a neuron that contain neurotransmitters, Wharton said.
The researchers also found that the mutants had less neural activity through a technique called electrophysiology, which is used to “measure several electrical features of cells,” such as neurotransmitter release and action potentials, said Aaron Held Ph.D.’19, lead author of the study. All electrophysiology work was conducted in collaboration with the Lipscombe Lab, he added.
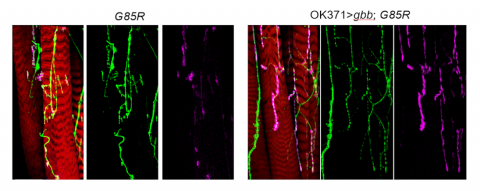
Furthermore, researchers tested BMP, a naturally occurring protein that has been shown to have a variety of effects including cell growth, to determine if it could “suppress motor defects observed in [the] mutants,” according to the study. About 9 percent of the mutants were able to leave their shells with BMP activation, and the frequency of synaptic activity and the number of synaptic boutons were almost completely restored with BMP activation.
The researchers then examined the motor function of the larvae, which were slightly impaired but not to the degree that the researchers expected from the pharates’ severe dysfunction, Wharton said. They crawled less and had longer but fewer waves of muscle contraction, according to the study. BMP activation led to shorter and more frequent waves of muscle contraction in the mutants, restored to the level exhibited by control flies. Additionally, some of the larvae became pharates that eventually eclosed.
Unlike the pharates, the structure and function of the neuromuscular junctions, or the synapses between motor neurons and muscles, of the larvae were not significantly different from those in their control counterparts. Held found that the characteristics of the action potentials leading up to a muscle contraction were also similar between mutant larvae and control larvae.
“That told us that the functionality of the synapse was normal when the motor neuron sends a signal,” Wharton said.
Held then used an electrophysiology technique called patch clamping to monitor the signals received by the motor neurons. While he did find that the motor neurons were slightly less excitable, it still didn’t seem to explain the differences in motor function, Wharton said.
Wharton Lab / Brown University
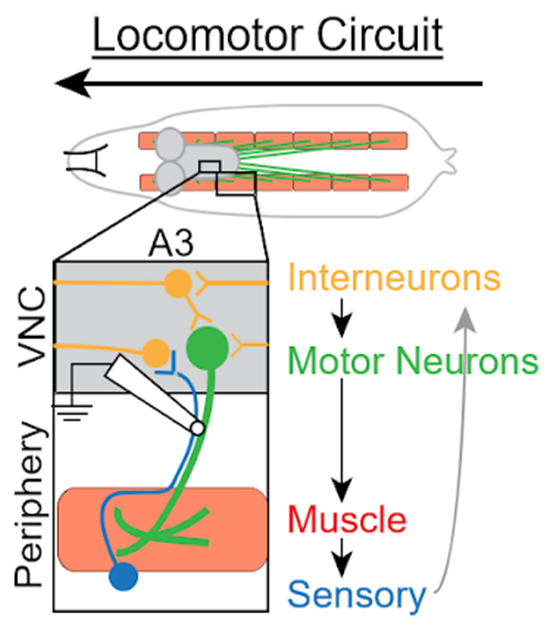
Having examined all the ways in which motor neurons could be deficient, the researchers turned to other parts of the circuit, Wharton said. While motor neurons relay signals from the central nervous system to contract muscle fibers, sensory neurons perceive the status of muscle contraction and communicate this information back to the central nervous system. Interneurons integrate these signals, which are then sent to motor neurons to complete the circuit, she said.
The researchers used a technique which allowed them to record neural activity from the intact motor circuit. They compared recordings from the intact motor circuit with those from flies in which the sensory axons mediating feedback to the central nervous system had been severed. The results indicated that signals from the periphery to the motor neurons were abnormal in animals carrying the ALS mutation, and that a dysfunction in this sensory feedback impacted circuit output, Wharton said.
To extend this line of research, Wharton and her lab members are working on understanding the exact mechanism behind the sensory feedback dysfunction. Whether there is impairment in the ability of sensory neurons to transmit information, impairment in the ability of interneurons to receive and integrate information, or a combination of both processes is yet to be determined.
Ideally, doctors will one day be able to test patients for “mutations that indicate they are high-risk for getting the disease, and then treat them by providing a therapeutic that would prevent a dysfunction so that the motor neuron would never decline,” Wharton said.
Researchers could also evaluate sensory neurons and interneurons for biomarkers of ALS, as an early diagnosis may result in more effective interventions, she added. Additionally, they hope to explain how activating BMP signaling helps to alleviate these adverse effects. Identifying “the cellular pathways regulated by BMP signaling [and] extending those findings to rodent models or patient derived neurons will be important for future development of therapies,” Wharton said.
Other researchers involved in the study include Paxton Major ’16; Asli Sahin Ph.D.’15; Robert Reenan, professor of biology; and Diane Lipscombe, director of the Carney Institute and professor of neuroscience.
The study was funded by the National Institutes of Health, ALS Finding a Cure Foundation, The Judith and Jean Pape Adams Foundation, and the Carney Institute.