The brain’s thalamus pipes sensory information — such as vision, hearing and touch — to the cortex, which controls the body’s responses to the sensory information. In a new study, published in Nature on July 22, researchers affiliated with the Carney Institute for Brain Science investigated how signals flow through a particular part of the thalamus called the thalamic reticular nucleus (TRN). They found two distinct groups of neurons in the TRN that work in ways appropriate for the information they are sending: one group sends strong but short-lived bursts to encode discrete events, while another sends weaker more sustained signals indicative of overall state.
“Our findings provide fundamental insights on how these two TRN subcircuits may process distinct classes of thalamic information,” said Rosa Martinez-Garcia, one of the first authors of the study and a postdoctoral research associate at Brown University.
Most sensory information destined for the neocortex — which is involved in higher-order brain functions such as sensory perception, cognition, language and attention — is conveyed through the thalamus. A goal in the neuroscience field, Martinez-Garcia said, is to understand how the TRN regulates information flow to and from the neocortex.
Prior to this study, scientists knew that information flows through multiple paths in the thalamus. Research had suggested that cross-talk between these different pathways in the thalamus help coordinate and control information. However, the new study suggests that each pathway functions independently. The researchers hypothesize that signals coming back from the cortex may coordinate these pathways, rather than direct connections in the thalamus between the pathways.
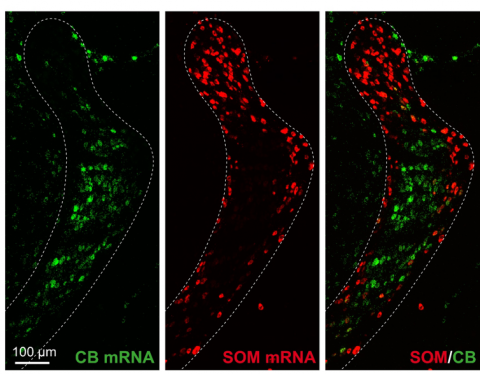
“Before, we knew that the information to the TRN was organized into sectors — visual, auditory and somatosensory — but beyond this we didn’t have a cohesive understanding of how cellular neurophysiological properties, molecular signatures of cell types, and incoming thalamic information may be aggregated within the TRN,” said Julia Zaltsman, one of the authors of the study and a Ph.D. student in the Neuroscience Graduate Program. “With this study, we’ve been able to show that the TRN is actually organized into these discrete subcircuits that dynamically and differentially regulate the information they receive.”
After identifying different neurons through genetics, the researchers examined the patterns of each cell type. One group of cells, found in the center of the TRN, were active in strong, short bursts, which quieted with sustained activity. Another group, located on the edge of the TRN, fired weaker but more sustained patterns. According to the researchers, the patterns of activity in central and edge TRN cells seem to match the type of information carried by the two subcircuits. The central circuits tend to carry information about specific events, while the edge circuits typically report information about general awareness state, where integration of multiple information sources over longer periods is required. The scientists observed this pattern first in the somatosensory area of the TRN, where touch information is processed.
“The visual sector of the TRN is similarly organized, suggesting that the principles we observe in the somatosensory sector may be a fundamental organizing principle of TRN circuitry,” says Martinez-Garcia, who graduated from Brown in 2019 with a Ph.D. in molecular biology, cell biology, and biochemistry. Three of the seven authors of this study are Brown University alumni.
Disturbances in the TRN have been linked to absence seizures, which are observed in some neurodevelopmental disorders. This work, the researchers said, may contribute to identifying therapy targets for absence seizures.
“We can now utilize genetic tools to selectively target and modulate core and edge subcircuits and study their roles in perception and behavior,” Martinez-Garcia said.
The research was funded by the National Institutes of Health (NS100016 and GM103645) and the National Science Foundation (1738633, 1058262 and 1632738).