
The study, published in eLife this summer, provides the first detailed molecular picture of projection neurons in the brain’s olfactory bulb. The olfactory bulb serves as the first relay for processing smells. Projection neurons are critical for routing information from the nose to higher-order brain regions, such as the olfactory cortex and the hippocampus.
“In this study, we provide the first detailed molecular description of the mouse olfactory bulb projection neurons,” said Zeppilli, a Ph.D. candidate in Brown’s Neuroscience Graduate Program and a recent recipient of a graduate award from the Carney Institute for Brain Science. “We explain the functional key gene regulatory networks of projection neurons together with their connectivity patterns, all critical features for defining a neuron’s identity.”
Zeppilli worked with scientists in Brown’s Fleischmann Lab, the Schaefer Lab at the Francis Crick Institute in London and the Crombach Lab at Inria in France. Here, she explains key findings and how the study advances the field, as well as how this hands-on scientific experience has been fundamental for her understanding of the brain and olfaction.
Tell us about your new study, what you found, and how you leveraged computational approaches to conduct your experiments.
In this study, we found distinct types of olfactory bulb projections neurons based on their molecular signatures and projection targets. We also found more subtypes based on the activity of their transcription factors, key genes for cell type identity and cellular function.
We developed a novel simulation method based on these transcription factor genes for integrating different sequencing experiments. This computational analysis was driven mainly by Robin Attey, an undergraduate student in our lab at that time. Our computational method simulates single nucleus-specific gene regulatory networks from bulk RNA deep-sequencing data, using gene regulatory network profiles from single-nucleus RNA sequencing-data as unit of analysis and integration. This method allowed us to predict that olfactory bulb projection neurons with different molecular signatures also preferentially project to distinct brain regions, a result that we have further validated experimentally.
What can we take away from this study, and how does it advance the field — particularly our knowledge of olfaction?
Our results suggest that subtypes of distinct projection neurons may share important functional properties, possibly blurring at the molecular level the traditional view into tufted and mitral cells as the two major types of olfactory bulb projection neurons. Through gene regulatory network interactions, gradual differences can be translated into the selective expression of functional genes, such as ion channels and receptors, providing a mechanism for generating and maintaining multiple distinct cellular phenotypes of a mature neuron.
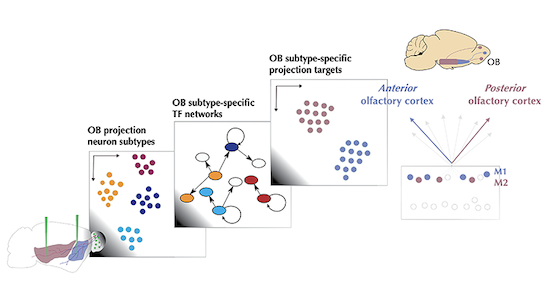
For the first time, and in contrast to the canonical view, our findings suggest a specificity in the projections to the anterior or to the posterior olfactory cortex for distinct molecularly-defined mitral cell types, one of the two main classes of olfactory bulb projection neurons. This suggests the possibility that independent channels have evolved to selectively direct information about distinct olfactory stimuli for specific behaviors, since the posterior olfactory cortex is believed to function more as an associative cortex.
Altogether, differential gene expression and connectivity patterns across distinct olfactory bulb projection neurons provide overlapping yet complementary axes for explaining, at least in part, their functional diversity — a model that can now be tested experimentally. Our study provides the knowledge and key tools for advancing our understanding of olfactory processing from the first relay, which is the bulb, to higher-order brain areas.
How has this hands-on research experience advanced your studies and your understanding of neuroscience?
For this study, we collaborated with data scientists, bioinformaticians and system neuroscientists with different training and research experiences. All of these collaborations and expertises were key to advance my training as a graduate student, and to develop skills that are crucial for a successful academic researcher career, including communication and organizational skills, and understanding and adapting to other people’s way of working.
This study has also been fundamental to expand my expertise and knowledge in olfaction — especially beyond the olfactory cortex, which is the brain area I mainly work on in the Fleischmann Lab. I learned a lot about the olfactory bulb and its connectivity to higher-order brain areas, a framework that has been investigated for many years.
What are your research interests, and what’s next in your professional career?
I am very interested in using olfaction as a model to study how different environments may have impacted sensory neural circuit organization during evolution. I am also fascinated by how the current revolution in genetics and cutting-edge technologies is diversifying and expanding neuroscience research, which is leading to more comparative approaches that allow us to better generalize working models.
After graduation, I intend to use my knowledge of neuroscience and my graduate training to continue a career as a scientist and contribute to increasing research diversity in academia.