The Robert J. and Nancy D. Carney Institute for Brain Science will provide $928,000 in seed funding for eight high-impact brain science research projects led by 12 Brown faculty members.
Now in its 11th year, the Zimmerman Innovation Awards in Brain Science jump-start promising, risky research, giving scientists the proof of concept they need to secure long-term funding from government, nonprofit or industry sources. It’s a formula with an impressive track record: as of fall 2024, brain science investigators who received a total of $4.9 million in innovation award funding had gone on to earn $166 million in external funding. This cycle of awards brings the institute’s total innovation award investment to $5,800,000.
This year, the institute will fund eight projects. Four projects will receive a total of $496,000 to address important questions from across the breadth of brain science. Another four will receive $432,000 to advance research that targets Alzheimer’s disease and related dementias.

Diane Lipscombe, Reliance Dhirubhai Ambani Director of the Robert J. and Nancy D. Carney Institute for Brain Science, said the awards support research that is exciting, timely and in need of proof of principle data.
“Each year I am more and more impressed by the creativity of our brain science community at Brown. This year, awardees are developing new tools to gain essential information for neurodegenerative diseases as well as applying the trans-Tango tool - developed with funds from this program - to study myelin, the electrical insulator in the nervous system,” Lipscombe said. “Other projects explore brain mechanisms that help humans solve learning problems in their social worlds, and the role of gut-brain communication in early-life stress.”
Bess Frost, Salame-Feraud Director of the Center for Alzheimer’s Disease Research, said Alzheimer’s disease won’t be eradicated through any one approach - which is why the innovation awards are so vital.
“These awards, all of which went to groups who were in some way collaborating across scientific disciplines, are coming at the research from many directions,” Frost said. “These big, bold approaches are a direct result of the diversity of the teams.”
The Carney Institute invests up to $100,000 per project for one year, renewable for a second year on a competitive basis. Projects led by a junior faculty member are eligible for an additional $32,000 in funding.
Below are the projects funded by this year's innovation awards.
A multimodal evaluation of adolescent cannabis use disorder: testing neurobehavior change over time
Investigator: Sarah Thomas, assistant professor of psychiatry and human behavior (research)
Adolescents who use cannabis progress more quickly toward cannabis use disorder than adults, but it is difficult to study neurobiological adaptations because the standard imaging technique, positron emission tomography (PET), isn’t safe for adolescents. Sarah Thomas has made a discovery that will enable her to safely study adolescents: teenagers who use cannabis have significantly lower brain tissue iron, measured with magnetic resonance imaging and representing dopamine neurophysiology, than teenagers who don’t. Through the innovation award, Thomas will use this unique imaging technique to track brain tissue iron levels in adolescents across a three month period to gather data about the development and progression of use disorders. “Our results have the potential to not only establish a safe way to study neuroadaptations in adolescent cannabis use–they could also help us understand how cannabis use's impact on dopamine may give rise to depressive and psychosis symptoms, which is a risk that happens when starting cannabis use during adolescence,” said Thomas.
Characterizing the contents of neural replay for social navigation
Investigators: Oriel FeldmanHall, Alfred Manning Associate Professor of Cognitive and Psychological Sciences, and Apoorva Bhandari, assistant professor of cognitive and psychological sciences (research)
Researchers have established that rodents “replay” their memories of traversing mazes while resting or sleeping to build cognitive maps that help them navigate those mazes in the real world. In a paper published in 2024, cognitive neuroscientists FeldmanHall and Bhandari established through human experiments and computational modeling that humans do something related but much more abstract: replay their memories of daily social interactions to build a map of social networks and successfully maneuver within society. Through the innovation award, FeldmanHall and Bhandari now seek to uncover the contents of this social neural replay–something that is extremely difficult to do because the neural signals involved are very hard to detect even with the most sophisticated methods. Using a new analysis technique they designed, the team will attempt to isolate the content of what human brains are replaying in fMRI data recorded during rest breaks subjects take in between performing a complex social navigation task. “Our project stands to provide a major contribution to our knowledge base of how people solve learning problems in their social worlds,” said the team.
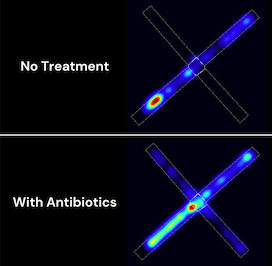
Microbial pathways of early life stress-induced anxiety
Investigator: Peter Belenky, associate professor of molecular microbiology and immunology
Can the gut-brain axis—the bidirectional communication between the central and enteric nervous systems—help explain how early-life stress leads to long-lasting anxiety? The Belenky Lab has leveraged a mouse model of early-life adversity to investigate this question. With the innovation award, the lab will explore how altering the gut microbiome—through treatments such as antibiotics—can influence anxiety-related behaviors. “Understanding the precise relationship between the gut microbiome and anxiety symptoms could pave the way for new microbiome-based interventions and diagnostics for stress-related disorders,” said Belenky.
Myelin trans-Tango: a new tool for labeling myelinated neurons
Investigator: Sonia Mayoral, Robert J. and Nancy D. Carney Assistant Professor of Neuroscience
Through the innovation award, Sonia Mayoral, an expert on myelin, will build a new, critically important tool. Myelin is a fatty substance made by glial cells that form around nerves. Scientists have known for a long time that myelin greatly increases the speed at which messages, in the form of electrical signals, pass between neurons. Only more recently have researchers realized that myelin also plays important roles in a host of other brain processes, including learning and memory, but they have not been able to study how myelin is involved because no tool for identifying myelinated neurons currently exists. Through a partnership with colleague Gilad Barnea, who used one of the earliest innovation awards to develop a suite of tools for tracing brain circuit communication called the trans-Tango toolkit, Mayoral will adapt aspects of trans-Tango to instead label myelinated neurons. “These mechanisms are extremely important to uncover since their discovery can lead to better therapies for neurodegenerative diseases where myelination is disrupted, like multiple sclerosis and Alzheimer’s disease,” said Mayoral.