This year’s Society for Neuroscience annual conference took place in San Diego, November 15-19. Students and postdocs from Carney-affiliated labs presented posters on aspects of their recent research. Below, five compelling presenters share their discoveries.

Presenter: Alice Lin, PhD student
Lab: Liu Lab
Presentation title: “Metabolic underpinnings of SLC13A5 epileptic encephalopathy”
Scientists had thought that SLC13A5 epileptic encephalopathy–a rare, genetic, early-onset epilepsy syndrome–was caused by impaired transport of citrate in the brain. Citrate is a molecule key to metabolism, and many therapies aiming to treat this type of epilepsy often focus on adjusting citrate levels. In her presentation, Lin shared findings that cast doubt on citrate as being at the root of the disorder. Lin was able to show that, in a mouse model of SLC13A5 epileptic encephalopathy, there was no connection between extracellular citrate levels and seizures.
“This information will help researchers rule out a potential mechanism of disease for patients. It could also help redirect research efforts toward other mechanisms that may be of greater translational relevance to treating SLC13A5 epileptic encephalopathy patients,” said Lin.
Funding: R01NS131865, F30NS141502, the Blavatnik Family Fellowship and the Sidney E. Frank Fellowship
____________________________________________________________________________

Presenter: Vanessa Rivera Núñez, postdoctoral scientist
Lab: Desrochers Lab
Presentation title: “Differentiating medial and lateral frontal cortex contributions to no-report visual abstract sequence tracking in humans”
Rivera Núñez’s presentation explored how a person’s brain detects when something in the world subtly breaks from patterns the person expects—even when the person is not consciously looking for such a change. In Rivera Núñez’s experimental task, participants were shown simple abstract sequences while undergoing fMRI, without being told to track or learn any rules. Occasionally the familiar pattern would change, allowing Rivera Núñez to pinpoint how the brain responded when a sequence felt “off” to the participant. One brain area, the rostrolateral prefrontal cortex (RLPFC), showed activity that increased with each step of a sequence that broke the learned rule.
“These findings suggest that RLPFC may serve as a kind of lead supervisor for abstract sequences, tracking whether incoming information follows the expected rule,” Rivera Núñez said. “Understanding this system may shed light on conditions in which flexible pattern monitoring breaks down, such as obsessive-compulsive disorder.”
Funding: NIMH Research Project Grant R01MH131615 and NIH-NIGMS Grant COBRE P20GM103645
____________________________________________________________________________

Presenter: Alisa Salazar, PhD student
Lab: Lipscombe Lab
Presentation title: “Developmentally-regulated alternative splicing of CACNA1A in cerebellum”
CACNA1A is a gene that governs a voltage-gated calcium channel–a tiny opening in the membrane of a neuron that lets calcium molecules flow through, enabling the neuron to communicate. Multiple genetic variants of CACNA1A that cause the channel to behave abnormally can lead to a variety of developmental disorders, including developmental delay, intellectual disability and epilepsy. In her presentation, Salazar revealed new knowledge about the gene, showing in a mouse model that alternative splicing, the process cells use to make different proteins from a single gene, plays an important role in healthy development. Prior to birth, and for the first several days of postnatal development, CACNA1A expresses an exon, a tiny piece of genetic code, as exon-37b. As the cerebellum rapidly matures in the first four weeks of postnatal development, CACNA1A begins to express that exon as exon-37a. When Salazar deliberately interfered with alternative splicing, causing the gene to express either only exon-37a or only exon-37b both before and after birth, she found that mice expressing only e37a are largely normal, whereas mice expressing only e37b have striking developmental and motor deficits, rarely surviving beyond weaning age.
“This insight could lead to gene therapies for children with CACNA1A developmental disorders, especially if we can identify additional splice forms that act in a protective way,” said Salazar. “The findings also underscore that even tiny changes in how a gene is spliced can have a big impact on how neurons function, shaping the way we grow, move and survive.”
Funding: NIH Grant GR5271591
____________________________________________________________________________
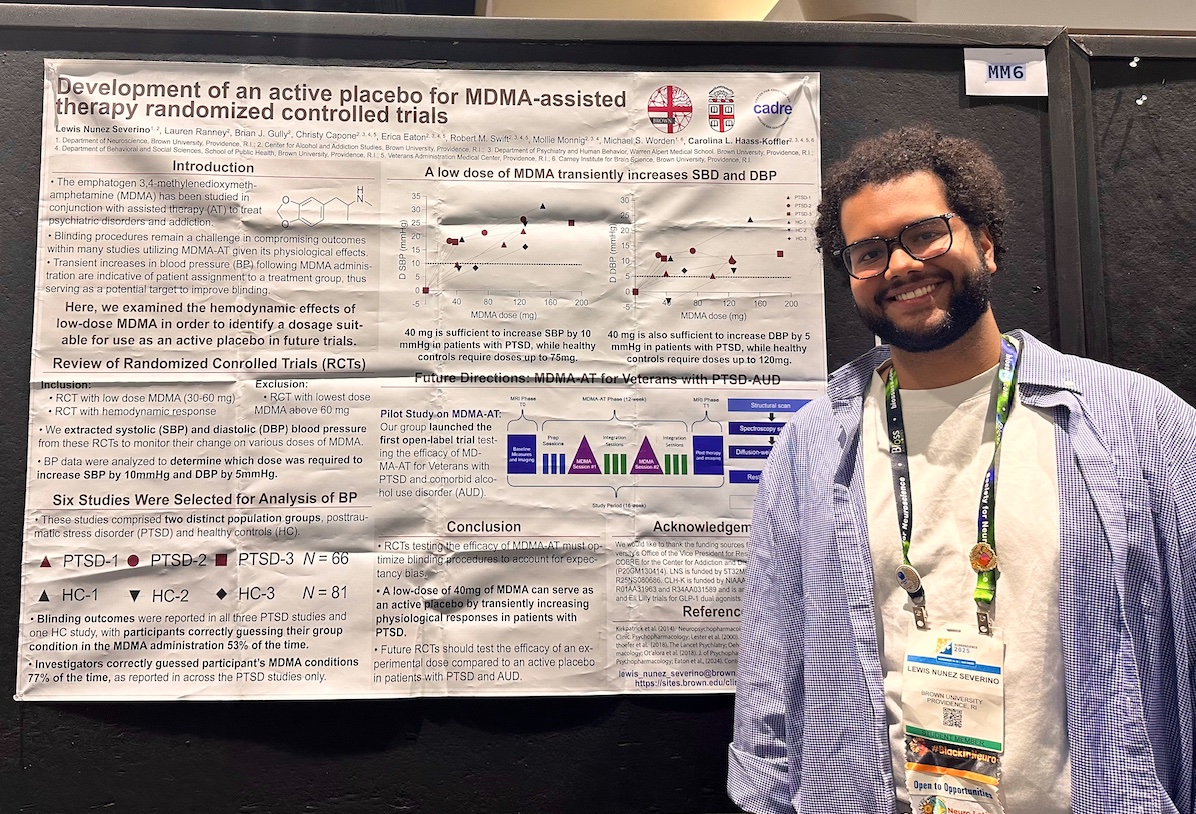
Presenter: Lewis Núñez Severino, PhD student
Lab: Haass-Koffler Lab
Presentation title: “Development of active placebo for MDMA-assisted therapy randomized controlled trials”
To properly conduct a placebo-controlled trial involving MDMA-assisted therapy, both participants and researchers need to be unaware of who is receiving a placebo versus the therapeutic dose of MDMA. Because it is commonly known that MDMA has psychological effects, participants can quickly catch on when they are in a control group. Because MDMA changes a subject’s blood pressure, researchers monitoring vitals and providing talk therapy can quickly pick up on this as well. Núñez Severino’s presentation showed that a low dose of MDMA (40 mg) can function as a placebo that doesn’t tip off subjects or researchers, since the low dose increases these telltale signs of taking the drug without being strong enough to have a health benefit.
“These findings will help improve the many studies being conducted on the efficacy of MDMA-assisted therapy on different populations, including our lab’s clinical trial with veterans experiencing comorbid post traumatic stress disorder and alcohol use disorder,” said Núñez Severino.
Funding: Work on MDMA-AT is funded by the COBRE Grant P20 GM130414 and the Brown University Division of Research. Travel to SfN was funded by the BP-ENDURE program (5R25NS080686).
____________________________________________________________________________
Presenter: Zachary Uttke, MD/PhD student
Lab: Frost Lab
Presentation title: “The nucleoside reverse transcriptase inhibitor 3TC ameliorates tau-mediated hippocampal neuroinflammation in tau transgenic mice”
Retrotransposons are silenced genes that, in Alzheimer’s disease, become active due to the abnormal build-up of the tau protein and, together with tau, contribute to neuroinflammation. Because retrotransposons are similar to retroviruses like HIV, it is possible that drugs designed to treat HIV may have benefits for patients with Alzheimer’s disease. In his presentation, Uttke described the initial results of a pilot study (conducted together with the Belfer Neurodegeneration Consortium) that repurposes the HIV drug 3TC to go after retrotransposons in mice engineered with pathological tau. The study found that, after treatment with 3TC, the hippocampi of the treated mice have reduced levels of gene transcripts related to neuroinflammation.
“The results of this pilot study will guide the Frost Lab’s next, more extensive study, where we will test multiple higher doses of 3TC in our mouse model,” said Uttke.
Funding: The Robert A. and Reneé E. Belfer Family Foundation, the Oskar Fischer Project and the Bowes Family Foundation